Now, the first thing we need you to do, Mr. Barish, is to go home and collect everything you own that has some association with Clementine.
Anything.
We’ll use these items to create a map of Clementine in your brain.
So, we’ll need photos, clothing, gifts, books she may have bought you, CDs you may have bought together, journal entries. We want to empty your home; we want to empty your life of Clementine.
After the mapping is done, our technicians will do the erasing…
This sounds like science fiction — and, of course, it is. It’s from the film Eternal Sunshine of the Spotless Mind. But the questions it raises about memory — about whether we could actually erase memories — those are very real.
Last week, we explored how memory works — how our brains encode, store and retrieve different types of memories. Our brain isn’t like a computer hard drive, where files are neatly stored and deleted. Instead, memories are reconstructed each time we remember them, with many brain regions working together to assemble the created experience.
This week, we’ll use Eternal Sunshine as a lens to examine whether we could manipulate memories in a similar way to how Joel has his memories manipulated in the film.
To find out, let’s first review the plot and then ask three questions:
Is it possible to prevent memories from forming?
Can we erase them once they’re formed? and
Could we implant entirely new memories that never happened?
Before we begin — if you haven’t seen Eternal Sunshine of the Spotless Mind yet, you really should. This is one of those movies that’s better experienced without knowing too much about it. And if you have seen it already, you’ll know it’s a fantastic movie to rewatch.
What is the Plot?
The film follows the relationship between Joel (Jim Carrey) and Clementine (Kate Winslet). But the story is told in a deliberately non-linear way.
The film begins with what seems to be a chance meeting between two strangers on a train to Montauk. But something feels off. Awkward even. And there are some strange gaps. Joel is confused by the name Clementine. It’s as if he’s never heard it before.
This opening scene is not the beginning of their story; It’s not the first time Joel and Clementine have met.
After the opening scene, the timeline shifts and we learn that Joel and Clementine have already been in a relationship. And it ended badly. Joel has discovered that his now ex-girlfriend Clementine has undergone a procedure at Lacuna Inc. to erase all of her memories of their relationship.
Devastated, Joel decides to undergo the same procedure. As the technicians systematically erase his memories, Joel begins experiencing these memories one last time. Starting with their bitter end and working backwards through their relationship, he experiences the fights, the joy, and the tender moments again — and he realises (possibly too late) that he doesn’t want to lose these memories after all.
The film’s premise taps into something deeply universal about the human experience of pain and memory.
Who hasn’t wished they could erase a painful memory? The devastating heartbreak of a failed relationship. The grief of losing a loved one. The shame of an embarrassing moment that keeps you awake at night. These deeply human desires to escape our painful memories make Eternal Sunshine’s premise so compelling — not just as science fiction but as a mirror to our own longings and fears about our memories.
But is such precise memory manipulation actually possible?
Q1: Could we prevent memories from forming?
The year is 1967. A goldfish swims lazily in a two-sided tank.1
As Mr. Goldfish is exploring the south end of his tank, the water around him suddenly brightens. He continues swimming, unbothered by the light. But twenty seconds later — ZAP! A mild electric shock jolts through the water. The shock doesn’t hurt, but it’s not exactly pleasant either. So, he darts over a barrier into the darker north side of the tank.
The peace, unfortunately, doesn’t last long for Mr. Goldfish. The north end of the tank is soon lit up. And again — ZAP! So, back to the south side, he goes.
Over the next forty minutes, Mr. Goldfish learns something important: every time the light comes on, he should swim to the other side of the tank to avoid being shocked.
Mr. Goldfish is one of dozens of goldfish swimming in tanks in a University of Michigan lab. Little do these fish know, they’re about to help scientists answer a mysterious question about how our memories work: When do experiences become permanent memories? And, could we prevent those memories from forming?
Before 1967, scientists had a hunch that whether an experience turned into a long-term memory or not, had a lot to do with proteins.
Inside every cell in our brains (including neurons), we have tiny molecular machines called ribosomes. These ribosomes work like assembly lines, linking together amino acids to form proteins — this process is called protein synthesis. Proteins are essential for neurons — they allow neurons to communicate and form new connections with other neurons. No proteins — and neurons can’t do what they usually do.
The scientists wondered: If protein synthesis is crucial for neurons to form new connections, what would happen if they blocked protein synthesis right after learning? Without the ability to make new proteins, neurons couldn’t communicate or create new connections. Would this prevent memories from forming altogether?
To test this idea, the scientists at the University of Michigan used an antibiotic called puromycin. Puromycin stops ribosomes from making proteins.
The researchers had a simple but clever plan: teach the goldfish a lesson, then use puromycin to block protein synthesis at different times after learning. A third of the goldfish were injected with puromycin immediately after training, another third were injected 30 minutes later, and the final third an hour after training.
When tested three days later, the results were striking: Fish that received puromycin immediately after training did not swim to the safe side of the tank when the light came on. They had forgotten what they had learned — it was as if the training had never happened. Fish that received the injection an hour later remembered perfectly well. And those injected at the 30-minute mark showed partial memory loss.
What the scientists had discovered was a crucial window. Somewhere between 0 and 60 minutes after learning, memories are consolidated — at least in goldfish. The timing was critical: block protein synthesis immediately after learning, and memories never form. Wait an hour, and the memory is fully consolidated — safe from the effects of puromycin.
For decades after this discovery, most scientists believed that memories were fragile while being learned but became permanent once consolidated.
It would take another 33 years — and a curious neuroscientist named Karim Nader — to challenge everything we thought we knew about memory storage.
Q2: Can we erase memories once formed?
In 2000, a young scientist named Karim Nader, who had recently started working in Joseph LeDoux’s lab at New York University, walked into LeDoux’s office with what seemed like an absurd question:
Could we erase an existing memory?
The idea seemed ridiculous. LeDoux’s reply: ‘There’s no way that will work’.
Remember, at this time, scientists thought memories were more or less permanent once they were consolidated.
But Karim had a hunch. What if memories become fragile again when we retrieve them?
Against LeDoux’s advice, Karim decided to run the experiment anyhow — secretly.2
First, he taught rats to fear a tone by pairing it with a mild foot shock. A rat’s response to fear is to freeze. So when the rats started freezing when he played the tone, Karim knew they had learned the tone meant a shock was imminent.
He waited two months to ensure the memory was well-consolidated.
Then came the crucial test:
Karim played the tone, the rats displayed their freezing response, and then he injected anisomycin directly into their amygdala (the brain area known for its role in fear responses). Like puromycin, anisomycin works by blocking cells from making proteins.
The next day, when Karim played the tone, the rats did not freeze. They showed no fear of the tone — it was as if the memory had been erased.
To show this wasn’t just general brain disruption, Karim taught rats to fear two different tones. Then, during the erasing procedure, he gave the anisomycin when just one of the tones was played. The next day, the rats only forgot their fear of the targeted tone — the other memory remained intact.
Karim bounced back into LeDoux’s office with the news, ‘it actually worked!’
The finding was revolutionary. It suggested that the mere act of remembering makes memories vulnerable again. It provided more evidence that when we recall a memory, we aren’t simply reading it out like a computer file — we’re reconstructing it, and in doing so, we open a window where that memory can be modified or even erased.
Imagine what this might mean for the millions who suffer from PTSD — soldiers returning from war, survivors of assault, first responders haunted by tragic scenes — traumatic memories aren’t simply harmless afterthoughts. For so many, painful memories trap people in a cycle of fear, anxiety, and depression.
While the anisomycin used in the rat studies is too toxic for human use, researchers have found other drugs that might have similar effects in humans. Early clinical trials suggest that giving patients these drugs while they remember traumatic memories can help reduce their emotional impact.
But it’s important to understand what’s happening with this type of treatment. This isn’t like the complete memory erasure depicted in Eternal Sunshine of the Spotless Mind.
In Karim’s experiments, anisomycin was injected specifically into the amygdala — a brain region critical for fear responses. What was likely being disrupted was not the entire memory of the event but rather something more specific.
The rats likely still retained the memory of hearing the tone and may have even remembered the experience of being in the chamber, but the fear response connected to that memory was erased. We’ll come back to this point later on.
Q3: Could We Implant Entirely New Memories That Never Happened?
Let’s travel back to the 1960s when researchers at UCLA were making an extraordinary claim — one that seemed almost impossible to believe. While scientists at the University of Michigan were discovering ways to block memory from forming in goldfish, these UCLA researchers proposed something even more radical: they believed they could transfer memories from one animal to another.3
Their method was surprisingly straightforward. They extracted RNA (a type of molecular cousin to DNA) from rats that had developed a learned fear response, then injected that RNA into untrained rats. The astonishing claim was that the untrained rats appeared to inherit the learned behaviours of the trained rats.
This wasn’t just an isolated finding from one lab, either. Other research groups began reporting similar results, suggesting they, too, could transfer memories through RNA.
But as the saying goes, extraordinary claims require extraordinary evidence. And some scientists were simply unconvinced that the evidence was extraordinary enough. When they attempted to replicate these studies using more rigorous experimental controls, they failed to reproduce the original results.
A scientific consensus formed — there was no compelling evidence that memories can be transferred between animals. In fact, for decades afterwards, making claims about memory transfer became somewhat of a taboo topic in neuroscience. There has been some reemergence of this topic, but it remains controversial.4
While these early experiments on memory transfer failed to stand up to scientific scrutiny, they raised an intriguing question:
Could we artificially create new memories that never actually happened?
Half a century later, scientists finally had tools sophisticated enough to tackle this question. A revolutionary new method called optogenetics was making waves in the neuroscience community.
Optogenetics is kind of like having a remote control for the brain.
To set it up, scientists use genetic techniques to add a special light-sensitive protein into specific neurons in a mouse’s brain. Once these proteins are in place, the researchers can switch these neurons on and off simply by shining a light on them — just like flipping a light switch.
The trick is in determining which specific neurons to target.
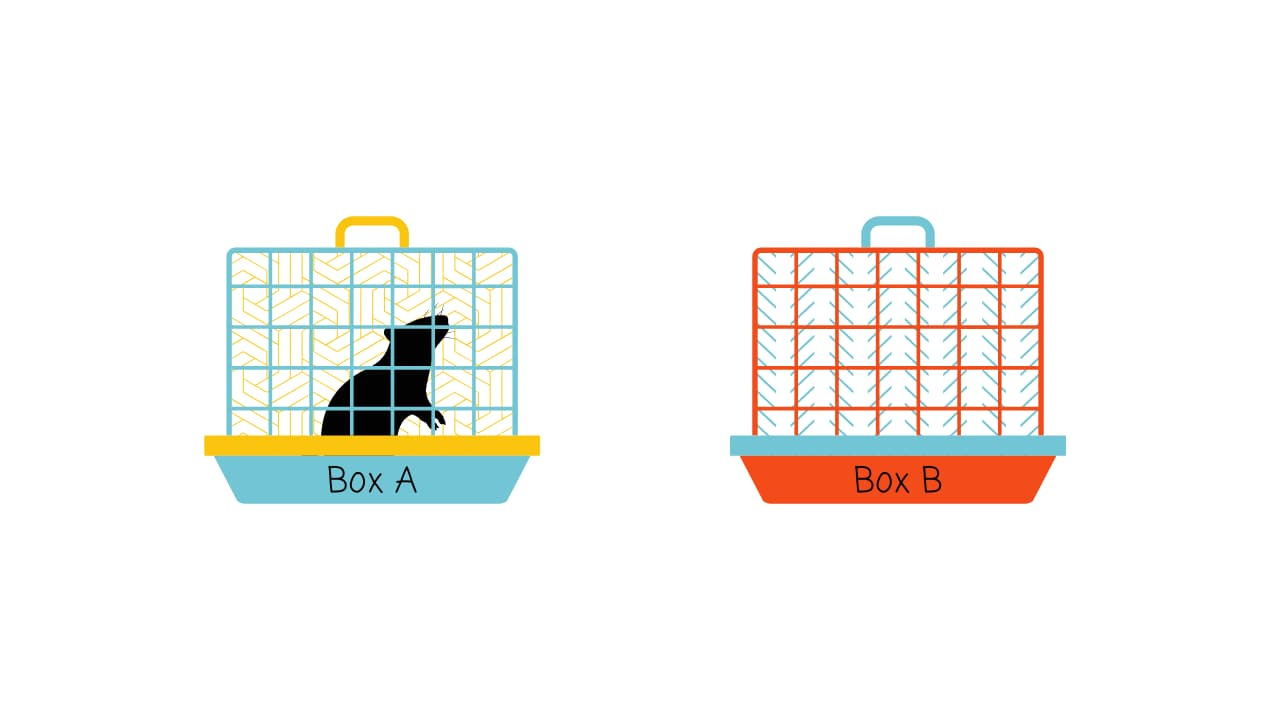
In one study, the researchers knew that when a mouse forms a memory, only a subset of neurons are active. So, they designed their system to add the light-sensitive protein only to neurons that were active when the mouse was in a particular box — let’s call it Box A.5
Box A had distinct features — specific visual patterns on the walls, a unique smell, and a particular texture on the floor — these are all things a mouse would use to recognise its environment.
The next day, they put the mouse in a completely different box — Box B. Here, they did two things simultaneously: they gave the mouse mild foot shocks while using light to switch on those Box A neurons. In other words, they artificially activated the neurons that had been active while the mouse was in Box A.
The researchers reasoned that by reactivating these neurons, they might make the mouse remember or even experience what they experienced while in Box A — but while they are actually in Box B receiving the shocks.
The result was striking. The next time the mouse was placed in Box A, it froze in fear — even though nothing bad had ever happened in Box A. By activating the Box A memory neurons during the fearful experience in Box B, the researchers had created a false memory. The mouse now seemed to remember being shocked in Box A, even though this had never occurred.
Timing of the light activation was crucial. False memories were only implanted when the neurons were activated during the shocks. If they activated the neurons before or after the shocks, no false memory was formed.
While these results are remarkable, it’s important to understand that there is a significant gap between learning that a tone means a shock is about to happen and the complex autobiographical memories we see manipulated in science fiction.
How Far Are We from Turning Science Fiction Into Science Fact?
In real life, scientists can create a simple associative memory — linking a particular context with a fear response — but they can’t implant specific complex memories that involve multiple sensations, emotions, and narratives, like memories of conversations, relationships, or life events.
Fear conditioning in animals involves relatively straightforward neural circuits, primarily in the amygdala. And this sort of memory is thought to be largely the unconscious type.
But human autobiographical memories, like Joel’s memories of Clementine, don’t exist in isolation. We can’t just go in and erase ‘Clementine’ from Joel’s brain as if there was a Clementine part that we could easily access. The neurons involved in Joel’s memory of Clementine are distributed across Joel’s brain — they interweave sensory details, emotions, semantic knowledge, and temporal sequences in ways we’re only beginning to understand.
But to complicate matters even more, the neurons involved in Joel’s memories of Clementine are likely active for other memories, too. For example, some neurons that fire when Joel remembers the sound of Clementine’s voice might also fire when he remembers his mother’s voice.
So, while it is remarkable that science can block, modify, erase and even artificially create memories, we’re still far from the kind of precise, selective memory manipulation depicted in sci-fi films and books.
But that doesn’t make movies like Eternal Sunshine any less fun to watch.
Next Week…
In this week’s and last week’s articles, we’ve seen how malleable our memories can be. All this talk of the fragility of memory might have us wondering how much of what we remember actually happened.
Next week, let’s explore the fascinating and sometimes disturbing science of false memories.
The implications are huge — could our sense of self, our relationships, and even our life decisions be shaped by memories that never actually happened?
Thank you.
I want to take a small moment to thank the lovely folks who have reached out to say hello and joined the conversation here on Substack.
If you’d like to do that, too, you can leave a comment, email me, or send me a direct message. I’d love to hear from you. If reaching out is not your thing, I completely understand. Of course, liking the article and subscribing to the newsletter also help the newsletter grow.
If you would like to support my work in more tangible ways, you can do that in two ways:
You can become a paid subscriber
or you can support my coffee addiction through the “buy me a coffee” platform.
I want to personally thank those of you who have decided to financially support my work. Your support means the world to me. It’s supporters like you who make my work possible. So thank you.
Agranoff, B. W. (1967). Memory and protein synthesis. Scientific American. DOI: 10.1038/scientificamerican0667-115
Nader, K., Schafe, G. E., & LeDoux, J. E. (2000). The labile nature of consolidation theory. Nature Reviews Neuroscience, 1(12), 216. DOI: 10.1038/35044580
Babich, F.R., Jacobson, A.L., Bubash, S. Jacobson, A.(1965). Transfer of a response to naive rats by injection of ribonucleic acid extracted from trained rats. Science, 149(3685), 757–759. DOI: 10.1126/science.149.3684.656
For example: Bédécarrats, A., Chen, S., Pearce, K., Cai, D., & Glanzman, D. L. (2018). RNA from trained Aplysia can induce an epigenetic engram for long-term sensitization in untrained Aplysia. Learning & Memory, 25(6), 307–316. DOI: 10.1523/ENEURO.0038-18.2018
The scientific consensus seems to be that while synaptic plasticity is important for memory, it is probably not sufficient to fully explain how memories are stored and maintained over long periods of time. This makes research like RNA transfer a fascinating area to keep our eye on.
Garner, A. R., & Fanselow, M. S. (2012). Generation of a synthetic memory trace. Science, 335(6075), 1513–1516. DOI: 10.1126/science.1214985









Great post.
A story from the earliest days of neuroscience suggests two things. First, that emotions exist in memories physically apart from declarative or episodic memory. And second, that emotional memories influence behavior unconsciously. More recent research supports this.
In 1911, a French physician named Edouard Claparede encountered a female patient with damage to both sides of her hippocampus. This kind of damage makes the consolidation of short-term memory into long-term memory impossible. His patient was incapable of creating any lasting episodic or declarative memories. Every visit Dr. Claparede made to his patient was like his first: he had to introduce himself to her as if it were the first time. Since her procedural memory was intact, she engaged in social rituals and shook hands with Dr. Claparede. One day, Dr. Claparede hid a sharp pin in the palm of his hand. When they shook hands, the pain of the prick startled her, but it was superficial and healed quickly. Because of her condition, she forgot about the incident. Despite this, when Dr. Claparede visited this patient again, she refused to shake hands with him and could not explain her own behavior. She had never been hesitant to shake his hand before. The act of shaking hands previously solicited a response with neutral or positive valence. Yet, now the doctor’s outstretched hand invoked a strong, negative feeling.
More on emotion and memory at https://tomrearick.substack.com/p/why-emotion-matters
So human memory has the destructive read problem! The earliest digital computers used ferrite core memory (basically rings of magnetic ferrite suspended on a 2D grid of wires) which equally had to be written again after every read.
[The most amazing thing about the movie is that it WASN'T based on a novel by Philip K. Dick!]